If you've been researching compounded tirzepatide in California, you may have encountered conflicting information online. Here's what you need to know: compounded tirzepatide is no longer available in California or anywhere in the United States as of March 19, 2025.
This guide explains what happened, why the change occurred, and what legitimate options remain for California residents seeking tirzepatide for weight management.
What Happened to Compounded Tirzepatide?
The FDA previously allowed compounded versions of tirzepatide during periods of supply shortage. This temporary authorization ended following an enforcement action in March 2025.
The decision was based on several factors:
- Manufacturing capacity increased to meet demand
- Safety concerns about unregulated formulations
- Reports of adverse events linked to compounded products
- Lack of standardized quality controls across compounding pharmacies
When prescribed by a licensed medical provider, and when clinically appropriate, tirzepatide remains available through FDA-approved channels only.

Why the FDA Ended Compounded Tirzepatide
The FDA's decision centered on patient safety.
Key Safety Concerns
Compounded medications operate differently than FDA-approved drugs:
- Each pharmacy creates its own formulation
- No standardized manufacturing oversight
- Limited ability to verify exact dosing
- Potential for contamination or incorrect ingredients
- Difficulty tracking adverse events across providers
The FDA received over 300 reports of adverse events potentially linked to compounded tirzepatide products. These incidents highlighted the risks of unregulated formulations.
Supply No Longer Constrained
Eli Lilly, the manufacturer of FDA-approved tirzepatide, expanded production capacity in late 2024. With adequate supply of regulated products available, the temporary authorization for compounding ended.
Your Current Options in California
California residents have two FDA-approved tirzepatide medications available through licensed healthcare providers.
Mounjaro® (Tirzepatide for Type 2 Diabetes)
Mounjaro is FDA-approved for managing type 2 diabetes in adults.
Approved Use:
- Type 2 diabetes management
- Used alongside diet and exercise
- Administered as a once-weekly injection
Some healthcare providers may prescribe Mounjaro off-label for weight management when clinically appropriate. Insurance coverage varies based on the prescribed indication.
Zepbound® (Tirzepatide for Weight Management)
Zepbound received FDA approval specifically for chronic weight management.
Approved Use:
- Weight management in adults
- BMI ≥30 (obesity), or
- BMI ≥27 (overweight) with at least one weight-related condition
- Used with reduced-calorie diet and increased physical activity
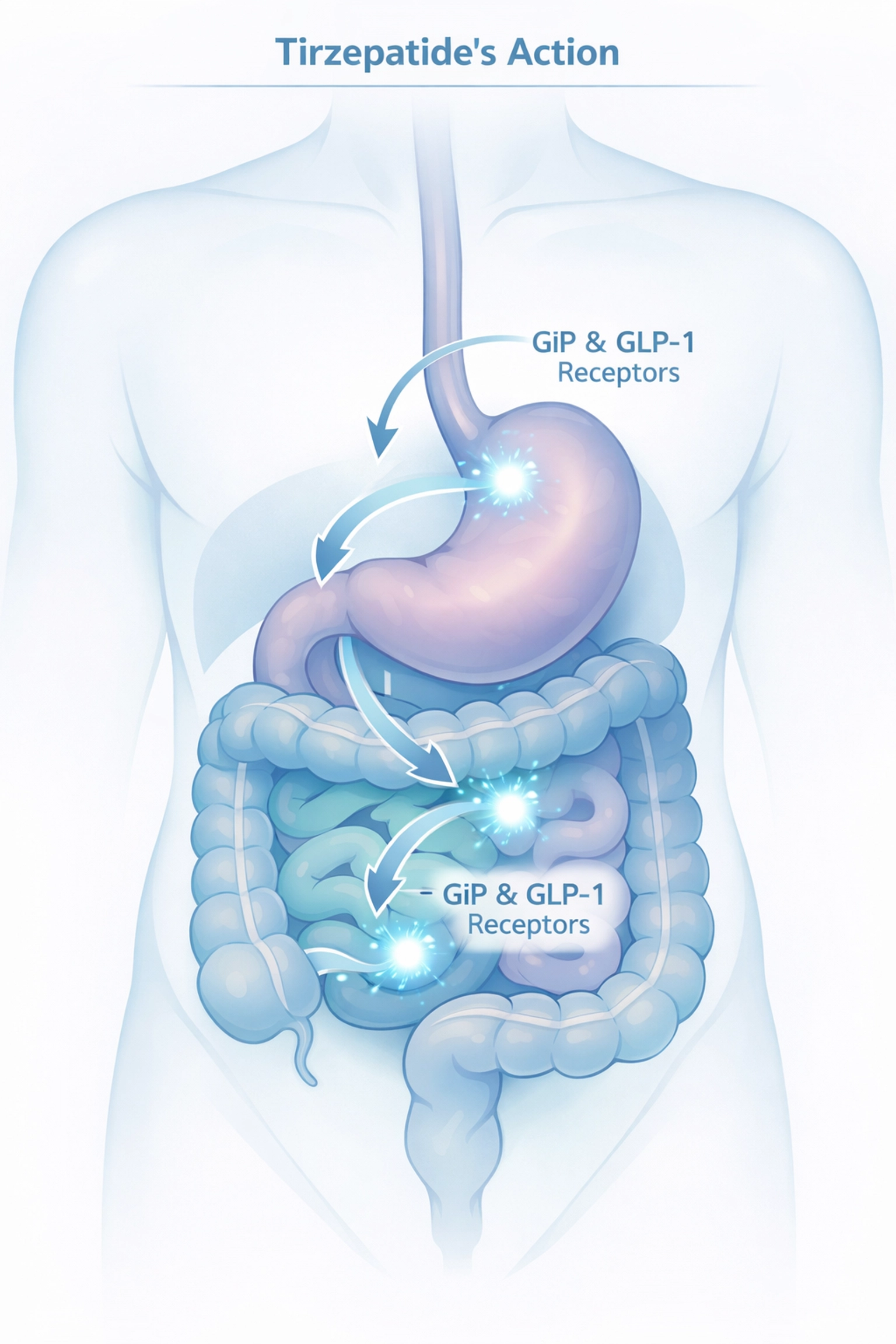
How Tirzepatide Works
Tirzepatide is a dual-action medication targeting two hormone receptors involved in blood sugar regulation and appetite control.
Mechanism of Action:
- Activates GIP receptors (glucose-dependent insulinotropic polypeptide)
- Activates GLP-1 receptors (glucagon-like peptide-1)
- Slows gastric emptying
- Reduces appetite signals to the brain
- Improves insulin sensitivity
The dual-receptor approach distinguishes tirzepatide from single-action GLP-1 medications like semaglutide.
When prescribed by a licensed medical provider, and when clinically appropriate, tirzepatide may support weight management goals as part of a comprehensive treatment plan.
Who May Be Eligible for Tirzepatide
Eligibility for tirzepatide depends on several medical factors.
General Eligibility Criteria
Healthcare providers typically evaluate:
- Body Mass Index (BMI)
- Existing medical conditions
- Previous weight management attempts
- Current medications
- Overall health status
- Contraindications
BMI Requirements for Zepbound
Standard criteria:
- BMI ≥30, or
- BMI ≥27 with weight-related comorbidities
Weight-related conditions may include:
- High blood pressure
- Type 2 diabetes
- High cholesterol
- Sleep apnea
- Cardiovascular concerns
Eligibility varies by individual. A licensed healthcare provider makes the final determination based on comprehensive medical evaluation.
Accessing Tirzepatide in California
Several pathways exist for California residents to access FDA-approved tirzepatide.
Traditional Healthcare Providers
In-person options:
- Primary care physician
- Endocrinologist
- Weight management specialist
- Obesity medicine clinic
Your existing healthcare provider can evaluate whether tirzepatide is appropriate and provide a prescription if clinically indicated.
Telemedicine Services
Online weight loss services offer convenient access to licensed providers who can:
- Conduct virtual consultations
- Review medical history
- Determine eligibility
- Provide prescriptions when appropriate
- Coordinate medication delivery
When prescribed by a licensed medical provider, and when clinically appropriate, telemedicine platforms like DrMed Health connect California patients with board-certified providers.
What to Expect During Evaluation
A typical consultation includes:
- Medical history review • Current health conditions • Previous treatments • Medications • Allergies
- Physical assessment • Height and weight • BMI calculation • Vital signs (if in-person)
- Discussion of goals • Weight management objectives • Lifestyle factors • Expected timeline
- Treatment planning • Medication options • Dosing schedule • Monitoring plan • Follow-up appointments
Start your assessment here to see if you may be a candidate for tirzepatide treatment.
Important Considerations for California Residents
Beware of Outdated Information
Some websites and marketing materials may still reference compounded tirzepatide availability. These sources contain outdated information.
Red flags:
- Sites promoting "compounded" versions
- Providers claiming to offer non-FDA-approved formulations
- Services without licensed prescriber involvement
- Products shipped from outside the United States
Legitimate providers only offer FDA-approved Mounjaro or Zepbound with valid prescriptions.
Cost Considerations
FDA-approved tirzepatide typically costs more than compounded versions did.
Factors affecting cost:
- Insurance coverage and copays
- Patient assistance programs
- Pharmacy pricing variations
- Geographic location
Many manufacturers offer savings programs for eligible patients. Discuss cost concerns with your healthcare provider to explore available options.
Ongoing Medical Supervision
Tirzepatide requires ongoing medical monitoring.
Typical monitoring includes:
- Regular check-ins with prescriber
- Tracking of side effects
- Weight and vital sign monitoring
- Lab work as indicated
- Medication adjustments based on response
When prescribed by a licensed medical provider, and when clinically appropriate, treatment plans include structured follow-up to ensure safety and effectiveness.

Frequently Asked Questions
Can I still get compounded tirzepatide with a prescription?
No. Compounded tirzepatide is not legally available in the United States as of March 2025, regardless of prescription status.
What if a website offers compounded tirzepatide?
Such offers violate current FDA regulations. Legitimate healthcare providers only offer FDA-approved formulations.
Is tirzepatide covered by insurance in California?
Coverage varies by insurance plan and prescribed indication. Zepbound for weight management may have different coverage than Mounjaro for diabetes.
How long does tirzepatide treatment typically last?
Treatment duration varies by individual response and clinical needs. Many patients remain on medication long-term with appropriate medical supervision.
Are there alternatives to tirzepatide?
Yes. Other GLP-1 medications are available, including semaglutide-based options. Your provider can discuss alternatives if tirzepatide isn't appropriate.
Next Steps for California Residents
If you're interested in exploring tirzepatide treatment:
- Get evaluated • Complete an assessment to determine potential eligibility
- Consult with a provider • Discuss your health history and goals with a licensed prescriber
- Understand your options • Learn about FDA-approved medications available to you
- Consider lifestyle factors • Tirzepatide works best with diet and exercise modifications
- Plan for monitoring • Commit to ongoing medical supervision throughout treatment
Learn more about how DrMed Health works for California residents seeking weight management support.
Medical Disclaimer
This article is for educational purposes only and does not constitute medical advice. Information about medication availability, eligibility, and treatment is subject to change and varies by individual circumstances. When prescribed by a licensed medical provider, and when clinically appropriate, tirzepatide may be an option for eligible patients. Individual results vary and are not guaranteed.
All prescription medications carry potential risks and benefits that should be discussed with a qualified healthcare provider. DrMed Health connects patients with licensed medical providers who make independent treatment decisions based on individual medical evaluations.
Eligibility criteria, insurance coverage, and medication availability may change. Always consult with a licensed healthcare provider for personalized medical guidance.
For more information about our services and credentials, visit our About page. DrMed Health complies with HIPAA privacy regulations and maintains strict confidentiality standards for all patient information.
Medically Reviewed: This content has been reviewed for accuracy by licensed healthcare professionals affiliated with DrMed Health's medical team.


